Health and Human Services (HHS) Secretary Tommy G. Thompson announced the approval on November 7, 2002.
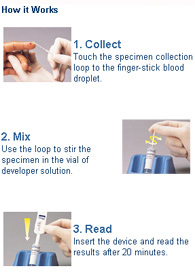
The test, which provides results in as little as 20 minutes, uses less than a drop of blood to detect antibodies to HIV. Manufactured by OraSure Technologies, Inc., Bethlehem, Pennsylvania, the OraQuick Rapid HIV-1 Antibody Test offers results with 99.6 percent accuracy.
If the OraQuick test gives a positive result, the findings must first be confirmed with an additional test, however, negative results do not require additional testing.
"I strongly urge the OraSure company to apply for a CLIA waiver," said Secretary Thompson. "If the FDA finds that the company's data proves that the OraQuick test is both easy and safe to use, it can get a CLIA waiver. Then the test could be given in many more health care settings, perhaps even administered by social workers in HIV counseling centers. But the process can't begin until OraSure applies for the waiver," he said. "Please apply now!"
About one fourth of the approximately 900,000 HIV-infected people in the US are not aware that they are infected, according to the Centers for Disease Control and Prevention (CDC).
"This test will be a great help in identifying pregnant HIV-infected women going into labor who were not tested during pregnancy so that precautionary steps can be take to block their newborns from being infected with HIV," said FDA Deputy Commissioner Dr Lester M. Crawford. "It will also be a critical resource in helping identify HIV infection in health-care and emergency workers who are accidentally exposed to HIV-infected blood while doing their job."
